Eczemol®
Pharmacological class: Homeopathic drug. NDC# 61480-127-05Eczemol® is indicated for the treatment of mild, moderate and severe eczema/atopic dermatitis in adult patients. It has been found to work well with a variety of combination therapies (see efficacy section).
Description: Eczemol® is a biochemical homeopathic medication indicated for the treatment of eczema. The active ingredients in each Eczemol® tablet consist of the following:
- Potassium Bromide (Kali Bromatum 1X)
- Sulphur 1X
- Nickel Sulphate (Niccolum Sulphuricum 1X)
The drug ingredients are listed in the Homeopathic Pharmacopoeia of the United States (HPUS).
Eczemol® demonstrates significant improvement in at least 4 weeks with or without the use of other oral and/or topical therapies.
A total of 41 adult patients with eczema/atopic dermatitis were studied in an open-label retrospective, cohort study examining the efficacy of Eczemol® when used alone or in combination with other oral and/or topical therapies over a significant course of at least 4 weeks treatment.
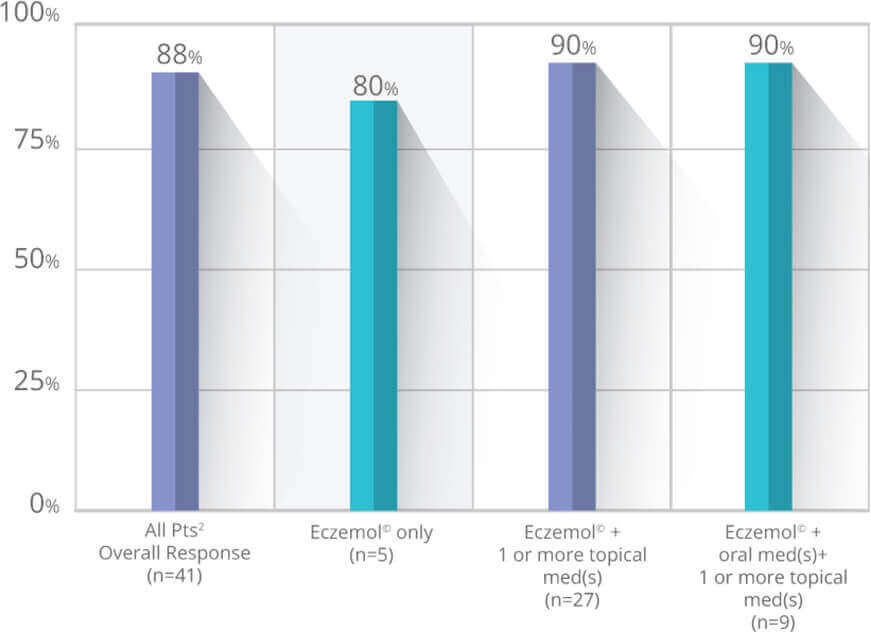
Before
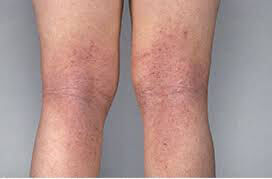
After 4 Weeks
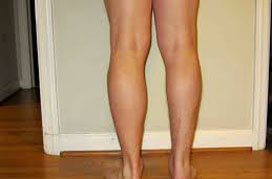
This is not an actual patient. Individual Results May Vary.

Psorizide® Ultra
Pharmacological class: Homeopathic Drug. NDC# 61480-124-05Psorizide® Ultra is indicated for the treatment of moderate to severe eczema, atopic dermatitis, seborrhea and seborrheic dermatitis. It has been found to work well with a variety of combination therapies.
Description: Psorizide® Ultra is a biochemical homeopathic medication indicated for the treatment of eczema and seborrhea. The active ingredients in each Psorizide® Ultra tablet consist of the following:
- Potassium Bromide (Kali Bromatum 1X)
- Nickel Sulphate (Niccolum Sulphuricum 1X)
- Zinc Bromide (Zincum Bromatum 4X)
These drug ingredients are listed in the Homeopathic Pharmacopoeia of the United States (HPUS).
Psorizide®Ultra:
Dosage Form: Oral 300 mg scored tablet. May be swallowed whole, chewed or dissolved in the mouth and swallowed.
Dosage & Administration:
| Pounds | Starting Dose | Maximum Dose |
| 50 – 100 | ½ Tablet | 1 Tablet |
| 100 – 150 | 1 Tablet | 2 Tablet |
| 150 – 200 | 2 Tablets | 4 Tablet |
| Over 200 lbs. | 3 Tablets | 6 Tablet |
Increase dose only if needed on a monthly basis up to the maximum daily dose. Treatment dose and duration depend on the individual
Maintenance Phase: In order to maintain symptomatic relief, medication may be continued at the same or reduced initial phase dose level.
Absorption of nickel sulphate is variable among individuals. For maximum nickel absorption, tablets should be taken orally at the beginning of the day or any convenient time after having taken nothing but water for at least 7 hours. Take nothing but water for one hour after taking medication to aid absorption.
Psorizide®Ultra may interfere with the effects of oral antibiotics by reducing their absorption. If a patient is taking Tetracycline Antibiotics, allow at least two (2) hours after taking Psorizide®Ultra before taking a Tetracycline Antibiotic. If a patient is taking Quinolone Antibiotics, allow at least six (6) hours after taking Psorizide®Ultra before taking a Quinolone Antibiotic.
In the setting of renal impairment, dosage should be adjusted and serum nickel and bromide levels should be followed. Steady state trough level should be drawn prior to ingesting the day’s dose after one week of dosing or at appropriate intervals. Target trough serum nickel level is 20 – 40 mcg/L. Warning: post dose peak levels are unreliable.


